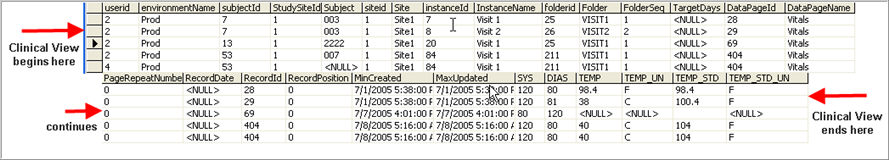
Each clinical view will return one column for each field in the form, plus additional columns if the field has units and/or standard value.
The Regular, Raw, and Lab Project Level Views will return the following fixed set of columns for the CRF Header information:
UserID
EnvironmentName
StudySiteId
SiteId
Site
SubjectId
Subject
InstanceId
InstanceName
FolderId
FolderSeq
TargetDays
FolderOID
DatePageId
DatePageName
PageRepeatNumber
RecordId
RecordPosition
RecordDate
MinCreated
MaxUpdated
Users can also add their own custom columns (up to 3), namely, Custom1, Custom2, and Custom3 to contain user defined contents.
The column names are derived from the field OID for each underlying field.
If a field has units and/or a standard value, the columns will have the following suffixes:
_UN for units
_ STD for standard value
_ STD_UN for standard units
If a field is flagged as Requires Translation, the clinical view containing that field will have two additional columns, one each for the translated field and the language of translation:
<FIELDOID>_USR for the translated field
<FIELDOID>_LNG for the language of translation
If a field has a Date or Number value, the columns will have the following suffixes:
_RAW for raw date columns (character date value, as entered by the user)
_RAW for raw number columns (character value for all numeric fields - non-conformant data)
_INT for interpolated dates (when day or month is missing)
Note: If Clinical View Suffix for Year, Month or Day Component of Date Fields is set as NON-NULL, each date field will generate a separate column for the Year, Month and Day component of the Date. These columns will be of Type Integer, and will be blank or NULL if an "Unknown" value is entered for that component of the date.
When a Clinical View Suffix for a field is set as NULL the corresponding column will not be created in the clinical view.

Clinical View Settings can be configured in the Configuration module. For more information, please see Configuration Help.
Copyright © 2014 Medidata Solutions, Inc. All rights reserved.